Stanford study shows how modifying enzymes’ electric fields boosts their speed
A seemingly subtle swap of metals—substituting a zinc ion with a cobalt ion—and a mutation ramps up the overall electric field strength at the active site of an enzyme, Stanford scientists find. The result is a predictably modified enzyme that works an astonishing 50 times faster than its unmodified analog.
Stanford researchers have demonstrated a way to dramatically speed up the reaction rate of an enzyme, a finding that could pave the way to designing ultra-fast synthetic enzymes for a range of industrial and medical uses.
Honed over billions of years of evolution, biological enzymes are marvels of chemistry. These specialized proteins serve as catalysts for accelerating chemical reactions essential to life as well as processes used in the food, pharmaceutical, and cosmetic industries.
Ever since enzymes' discovery nearly two centuries ago, scientists have sought ways to make them even faster. Most fabricated enzymes, though, have failed to match the lofty efficiency standards of nature-made varieties. And even where some successes have been realized through directed evolution, a protein engineering method that mirrors nature's trial-and-error approach, these successes so far have been by chance, not because of a deeper understanding of how enzymes work or could be modified to work more swiftly.
Now, in a new study, researchers at Stanford’s School of Humanities and Sciences and SLAC National Accelerator Laboratory have debuted a modified enzyme that works an astonishing 50 times faster than its unmodified analog. The findings derive from pioneering research at the university regarding electric fields generated at "active sites," the pocketlike places where revved up chemical reactions occur. Based on this concept, the researchers tweaked the chemistry of the active site, boosting its electric field strength and specificity to deliver the zippy results.
"We have improved a natural enzyme's activity through an understanding of how changing atoms at the active site can intentionally enhance electric fields," said Chu Zheng, co-lead author with Zhe Ji of the study published Aug. 10 in the journal Nature Chemistry.
Zheng conducted the work as a graduate student in the lab of Steven G. Boxer, the Camille Dreyfus Professor of Chemistry in H&S. Zheng is now a postdoctoral scholar in the lab of Peter Kim, the Virginia and D.K. Ludwig Professor in Biochemistry. Ji, a former Stanford postdoctoral scholar in Boxer’s lab, is now an assistant professor at Peking University.
"With this study, we have succeeded in making an enzyme that works better—significantly better, in fact—than a natural enzyme and in a rational, predictable way," said Boxer, the chair of the Department of Chemistry and senior author of the study. "Our study presents a new paradigm for designing biological enzymes and should be readily applicable to designing non-biological catalysts as well."
Revamping an enzyme
Ten years ago, the Boxer Lab began developing methods for probing the electric fields produced by the highly organized 3D atomic structures of enzymes' active sites and connecting these fields with the rates of the reactions catalyzed by the enzyme. Lab studies since have borne out how the electrostatic interactions driven by these fields contribute heavily to the bespoke environments in active sites, where specific substrate molecules fleetingly bind, react, and transform into new molecules.
While examining such activity in an enzyme called horse liver alcohol dehydrogenase, Zheng and colleagues made a serendipitous discovery. The researchers noticed that the active site produced a stronger electric field when swapping out an amino acid, serine, for a similar amino acid called threonine. The hydrogen atoms in these amino acids form hydrogen bonds—often an essential interaction in enzyme catalysis.
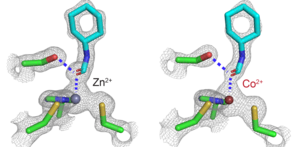
Zheng, an expert in the chemistry of metals, delved further into tweaking other active site ingredients. In many enzymes, zinc (Zn) ions play a pivotal role in catalysis, forming a coordination complex (a central atom or ion surrounded by bound molecules or ions) within the active site. To wring out greater performance, Zheng and Ji tried replacing the zinc ion in the horse enzyme's active site with atoms of other metals.
As it turned out, subbing in cobalt (Co) for Zn looked promising in ramping up the overall electric field strength. Both elements in this coordination complex context appear in a catalytic ion state as Zn2+ and Co2+, meaning they have a net charge of +2. To incorporate this alternative metal, the researchers developed a method to replace the native Zn2+ with Co2+, but swapping metals in the enzyme could alter its overall structure and, as a consequence, its function.
To verify that the arrangement and bonding of atoms in their newly honed enzyme was unaltered, the Boxer Lab team enlisted the assistance of Irimpan Mathews, a lead scientist at SLAC. Mathews measured the 3D structure of each modified enzyme using X-ray crystallography and confirmed that the modification left the overall structure unaffected.
The researchers then measured the modified electric fields in the modified active sites via vibrational Stark effect spectroscopy, a method pioneered in the Boxer Lab. This technique measures the vibrational frequencies in molecules based on the wavelength of infrared light absorbed by their chemical bonds. Shifts in these vibrational frequencies reveal information about the electric fields present. The researchers also created computer models to investigate their theory that the alt-enzyme would have enhanced performance.
Proof-of-principle enzyme enhancement
With the adjusted enzyme thus prepped and the electric field measured, the researchers put it through its paces and measured its speed. Reassuringly, just as predicted in the researchers' computer models, the modified enzyme performed about 50 times faster than its natural counterpart.
To build on these proof-of-principle results, the researchers plan to continue devising enhanced enzymes and characterizing their electrostatic interactions.
"We have shown that hydrogen bonds and metal coordination, two entirely distinct yet essential forces in enzyme catalysis, can be unified via electric fields, a fundamental physical quantity," Zheng said.
"And because we're dealing with physical quantities, we can add or subtract them to achieve desired electric field strengths and then predict the resulting effect on the rate,” Ji said. “That's a very high level of control over enzyme performance."
"The approaches we demonstrated in this study could prove very effective in custom tailoring more powerful catalysts in the future," Boxer added. "We hope this study provides a basis for more rational design of better catalysts."
Acknowledgements
This work was funded by the National Institutes of Health. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program was funded by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences